Oxidation: An Introduction to Lipid Oxidation (Part 1)
October 17, 2025
Oxidation at the Chemical Level
Lipid oxidation, quite literally, refers to a reaction in which oxygen binds to lipids.
Let’s take a closer look at this process from a chemical perspective.
Lipids commonly found around us include phospholipids, triacylglycerols, and cholesterol esters.
Phospholipids constitute the structure of cell membranes, triacylglycerols serve as energy reserves within the body, and cholesterol esters circulate as carriers of energy.
These lipids are not only biosynthesized within the body but are also obtained from our diet.
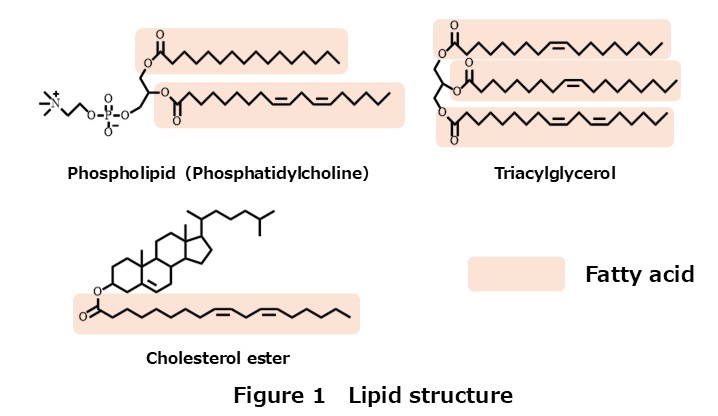 Let’s take a look at Figure 1.
These lipids contain a structure known as a fatty acid (although some lipids lack it).
Fatty acids include well-known molecules such as oleic acid, linoleic acid, eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA).
Phospholipids have two fatty acids attached, whereas triacylglycerols have three.
Even among phospholipids of the same class, the types and combinations of constituent fatty acids vary widely.
Therefore, lipids can be regarded as an extremely diverse group of molecules.
Let’s take a look at Figure 1.
These lipids contain a structure known as a fatty acid (although some lipids lack it).
Fatty acids include well-known molecules such as oleic acid, linoleic acid, eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA).
Phospholipids have two fatty acids attached, whereas triacylglycerols have three.
Even among phospholipids of the same class, the types and combinations of constituent fatty acids vary widely.
Therefore, lipids can be regarded as an extremely diverse group of molecules.
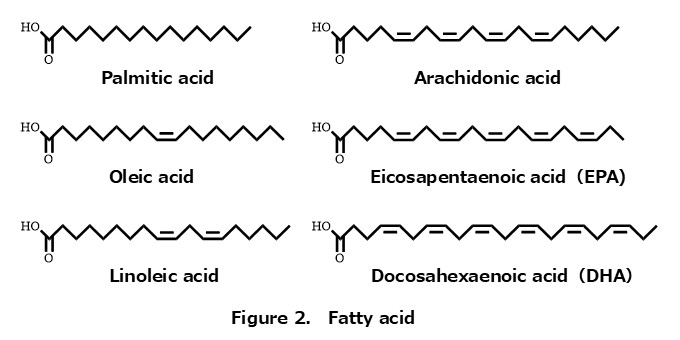 By the way, fatty acids differ in their number of carbon atoms (chain length) and double bonds, and these structural differences greatly influence the physical properties of lipids (Figure 2) — for example, their melting points.
From the perspective of oxidation, the more double bonds a fatty acid has, the more easily it undergoes oxidation.
You may have heard that DHA is beneficial for the brain and overall health; however, because DHA contains as many as six double bonds, it is extremely susceptible to oxidation.
This is why great efforts are made in the food industry to prevent its oxidation.
In the next section, I will discuss how these fatty acids actually undergo oxidation.
By the way, fatty acids differ in their number of carbon atoms (chain length) and double bonds, and these structural differences greatly influence the physical properties of lipids (Figure 2) — for example, their melting points.
From the perspective of oxidation, the more double bonds a fatty acid has, the more easily it undergoes oxidation.
You may have heard that DHA is beneficial for the brain and overall health; however, because DHA contains as many as six double bonds, it is extremely susceptible to oxidation.
This is why great efforts are made in the food industry to prevent its oxidation.
In the next section, I will discuss how these fatty acids actually undergo oxidation.